rel-trial Clinical trial database
Database Description: The clinical trial database is curated from AACT initiative, which consolidates all protocol and results data from studies registered on ClinicalTrials.gov. It offers extensive information about clinical trials, including study designs, participant demographics, intervention details, and outcomes. It is an important resource for health research, policy making, and therapeutic development.
Database Statistics:
| Domain | Medical |
| Num of Tables | 15 |
| Num of Rows | 5,434,924 |
| Num of Columns | 140 |
| Starting Time | 2000-01-01 |
| Validation timestamp | 2020-01-01 |
| Testing timestamp | 2021-01-01 |
| Time window | 1 year |
Database schema:
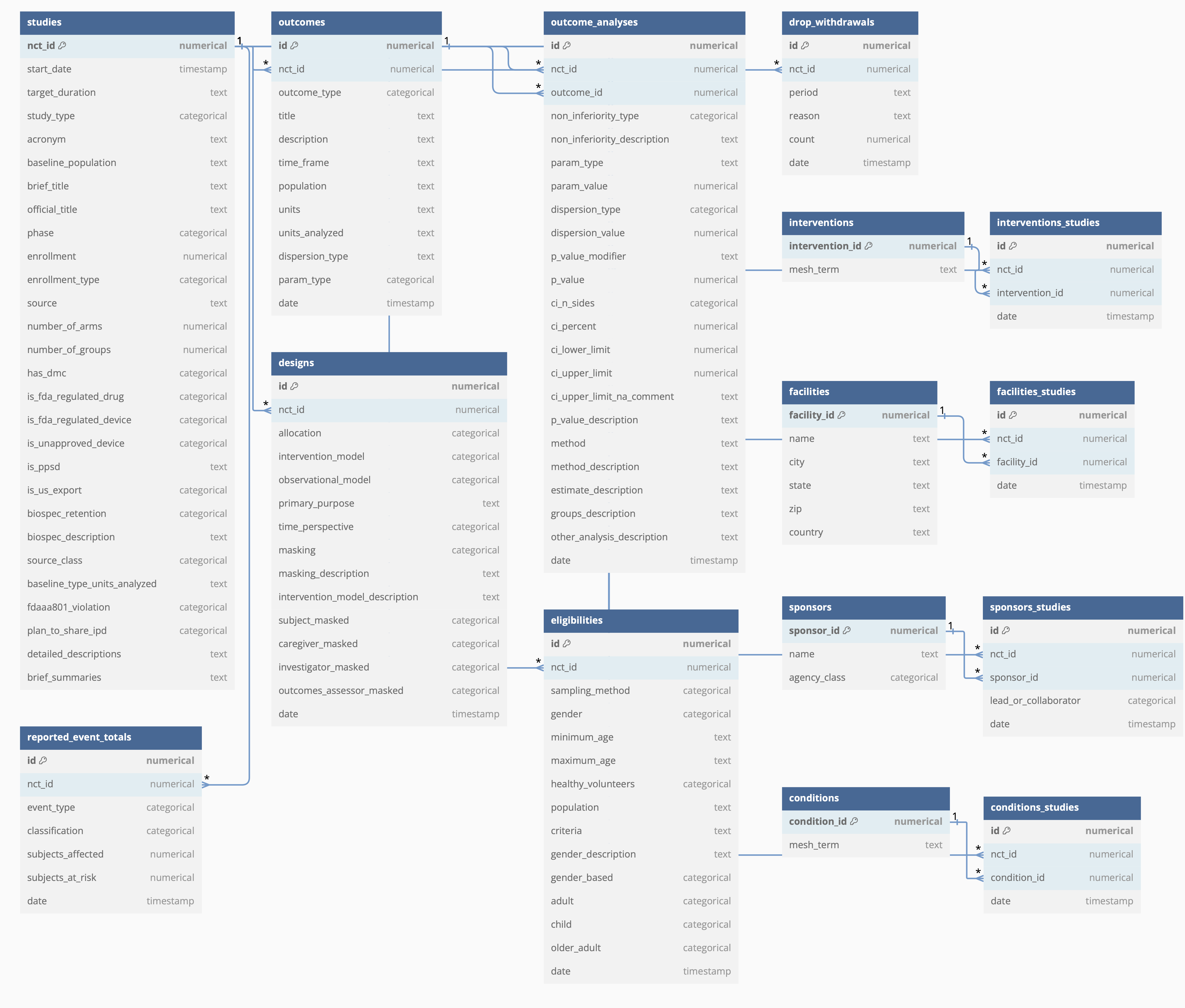
To load this relational database in RelBench, do:
from relbench.datasets import get_dataset
dataset = get_dataset("rel-trial")
References:
[1] Clinical Trials Transformation Initiative.
Dataset License: Not specified.
Entity Classification Tasks
study-outcome
Task Description: Predict if the trials will achieve its primary outcome (defined as p-value < 0.05).
Evaluation metric: AUROC
studies-has_dmc
Task Description: For each study, predict whether it has a data monitoring committee.
Evaluation metric: AUROC
eligibilities-adult
Task Description: For each study, predict whether it includes adult participants.
Evaluation metric: AUROC
eligibilities-child
Task Description: For each study, predict whether it includes child participants.
Evaluation metric: AUROC
Entity Regression Tasks
study-adverse
Task Description: Predict the number of affected patients with severe advsere events/death for the trial.
Evaluation metric: MAE
site-success
Task Description: Predict the success rate of a trial site in the next 1 year.
Evaluation metric: MAE
studies-enrollment
Task Description: For each study, predict the enrollment count.
Evaluation metric: MAE
Link Prediction Tasks
condition-sponsor-run
Task Description: Predict whether the sponsor (pharma/hospital) will run clinical trials for the condition (disease)in next year
Evaluation metric: MAP
site-sponsor-run
Task Description: Predict whether this sponsor (pharma/hospital) will have a trial in the facility in next year
Evaluation metric: MAP